Oncoscience
Cystadenofibroma and contralateral collision lesions: A unique ovarian case report
Naina Kumar1, Ashutosh Rath2, Aparna Setty1, Pooja T. Rathod1 and Jarathi Aparna1
1Department of Obstetrics and Gynecology, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana 508126, India
2Department of Pathology, All India Institute of Medical Sciences, Bibinagar, Hyderabad, Telangana 508126, India
Correspondence to: Naina Kumar, email: naina.obg@aiimsbibinagar.edu.in
Keywords: collision tumor; cystadenofibroma; mucinous; ovary; serous
Received: January 22, 2025
Accepted: March 24, 2025
Published: March 31, 2025
Copyright: © 2025 Kumar et al. This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Introduction: Ovarian cystadenofibromas are rare benign tumors, accounting for only 1.7% of benign ovarian neoplasms. Even rarer are ovarian collision tumors, with the coexistence of collision tumors and other benign ovarian neoplasms being exceptionally uncommon. This report presents a unique case of serous cystadenofibroma in one ovary, accompanied by collision features involving serous and mucinous cysts in the contralateral ovary. Case report: A 41-year-old woman presented with lower abdominal pain and swelling persisting for 2–3 months. Clinical evaluation of the abdomen revealed a mobile, non-tender, cystic mass resembling a 26–28-week gravid uterus, with no free fluid. Local and per speculum examinations showed a healthy vulva, cervix, and vagina, with a Pap smear negative for intraepithelial lesions or malignancy. A bimanual examination identified a mobile, multiparous uterus and a large (~15 × 12 cm), predominantly cystic lesion originating from the right adnexa and extending anteriorly and superiorly to the uterus. MRI findings confirmed these observations. Given the endometrial biopsy indicating endometrial intraepithelial neoplasia, the patient underwent an exploratory laparotomy with total abdominal hysterectomy and bilateral salpingo-oophorectomy. Histopathological analysis revealed serous cystadenofibroma in the right ovary and multiple serous and mucinous cysts in the left ovary, consistent with collision features. Additionally, the uterine endometrium showed hyperplasia without atypia. Conclusion: This case underscores the rare coexistence of a benign surface epithelial-stromal tumor in one ovary and collision features in the other. It emphasizes the importance of comprehensive evaluation, precise diagnosis, and timely surgical management to ensure optimal patient outcomes.
INTRODUCTION
Serous cystadenofibromas are rare benign ovarian surface epithelial-stromal tumors, accounting for approximately 1.7% of benign ovarian neoplasms [1, 2]. These tumors comprise dense fibrous stroma interspersed with epithelial cystic components [3]. They are classified into serous, endometrioid, mucinous, clear-cell, and mixed subtypes, with the serous subtype being the most common, representing approximately 75% of cases [4]. Typically, unilateral, serous cystadenofibromas are most frequently observed in women aged 15–65 years [1]. Clinically, these tumors are often asymptomatic and are incidentally discovered during routine gynecological ultrasounds. However, when symptoms do manifest, they may include lower abdominal pain, vaginal bleeding, or a palpable abdominal mass [4, 5]. Occasionally, symptoms such as metrorrhagia or signs of feminization may occur, likely due to tumor-induced hyperestrogenism. This hyperestrogenic state is thought to result from excessive hormone production directly associated with the tumor [6].
Collision tumors, on the other hand, are rare neoplasms characterized by the coexistence of two distinct tumors within the same organ without histological intermingling [7]. Although collision tumors can occur in various organs such as the stomach, liver, and lungs, their presence in the ovary is uncommon. Among ovarian collision tumors, teratomas are among the most frequent components observed. These tumors are typically unilateral, with sizes ranging from 5.5 to 200 cm, and are most commonly found in patients aged 17 to 66 years [8, 9]. Each component of a collision tumor develops independently without any direct connection, and its unique characteristics dictate its biological behavior [9]. The clinical presentation is often nonspecific, with many patients remaining asymptomatic. Some individuals, however, report symptoms such as intermittent abdominal pain, bloating, or frequent urination, which are typically due to the compression of adjacent structures by the tumor mass [10]. Rarely, symptoms such as ascites or abnormal uterine bleeding may also occur [11]. The biological behavior of these tumors is determined by the nature of their components. If one of the tumor components is malignant, it may exhibit aggressive features, including local tissue invasion, involvement of adjacent organs, and distant metastasis [10, 12].
This case report highlights a rare occurrence of serous cystadenofibroma in one ovary alongside collision features involving serous and mucinous cysts in the contralateral ovary. Such findings are exceptionally rare in the literature, emphasizing the importance of documenting and reporting these unique cases to enhance the understanding of their pathogenesis and clinical presentation.
CASE REPORT
A 41-year-old woman, para 3 live 2, presented to the Obstetrics and Gynecology outpatient department with a 2–3-month history of lower abdominal pain and swelling. She denied symptoms such as vomiting, appetite loss, weight loss, irregular menstrual cycles, dysmenorrhea, or heavy menstrual bleeding. Her last menstrual period was on November 18, with a regular menstrual cycle of 28–30 days and 2–3 days of bleeding. She had no significant medical history.
On examination, her body mass index (BMI) was 23.8 kg/m², and her vital signs were normal. Abdominal examination revealed a mobile, non-tender, cystic mass resembling a 26–28 weeks gravid uterus, with no free fluid detected. Local and per speculum examinations showed a healthy vulva, cervix, and vagina, with a negative Pap smear for intraepithelial lesions or malignancy. Bimanual examination revealed a mobile multiparous uterus and a large, predominantly cystic lesion (~15 × 12 cm) originating from the right adnexa, extending anteriorly and superiorly to the uterus. The left ovary was also enlarged and palpable.
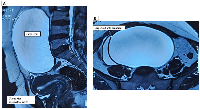
Abdominal ultrasonography showed a 68 × 44 × 30 mm uterus with an 8.9 mm endometrial thickness (ET) and a 14.3 × 11.8 × 13 cm anechoic cystic lesion in the right adnexa, compressing the bladder, suggestive of a complex ovarian cyst (serous/mucinous cystadenoma). The left ovary appeared normal. Tumor markers revealed CA-125 levels of 22.3 U/ml, HE4 levels of 52.7 pmol/L, and a Risk of Ovarian Malignancy Algorithm (ROMA) score of 21.6%. Magnetic Resonance Imaging (MRI) confirmed a multilocular cystic lesion (~14 × 14 × 8.2 cm) arising from the right ovary (ORADS III), with mild ascites and no significant lymphadenopathy (Figure 1A, 1B).
Anemia correction with two units of blood transfusion was performed (initial hemoglobin 7.5 g/dL). An endometrial biopsy revealed endometrial intraepithelial neoplasia (EIN). Following anemia correction, the patient underwent exploratory laparotomy with total abdominal hysterectomy and bilateral salpingo-oophorectomy. Intraoperatively, the right ovary had a 14 × 15 cm predominantly cystic lesion extending over the uterus, while the left ovary exhibited 2–3 cystic lesions (largest ~3 × 4 cm) (Figure 2A–2C). Minimal ascites (~100–150 ml) were noted, with cytology negative for malignancy. Other intra-abdominal structures, including the omentum, peritoneum, intestines, liver, and diaphragm, appeared grossly normal.
Gross pathology revealed the right ovary (14 × 13 × 8 cm) with two cystic cavities filled with serous fluid and thin walls (0.2 cm) with papillary excrescences. The left ovary (4.5 × 3.5 × 4 cm) contained multiple cysts, the largest measuring 3.5 cm. Microscopic examination confirmed serous cystadenofibroma in the right ovary and multiple serous and mucinous cysts in the left ovary, indicative of collision features (Figure 3A–3C). The uterus exhibited hyperplasia without atypia, while the cervix showed chronic cervicitis. Bilateral fallopian tubes, omentum, and peritoneum were histologically unremarkable.
Histopathological analysis confirmed the rare coexistence of serous cystadenofibroma in one ovary and collision features in the contralateral ovary. The patient tolerated the surgery well, with an uneventful postoperative recovery. Sutures were removed, and she was discharged in stable condition on the eighth postoperative day.
DISCUSSION
Surface epithelial-stromal tumors of the ovary are classified into five primary subtypes: serous, mucinous, endometrioid, clear cell, and transitional cell, with the possibility of mixed or combined forms among these categories [13]. According to the World Health Organization (WHO) classification, benign ovarian tumors encompass a diverse range of entities, including papillomas, cystadenomas, adenofibromas, cystadenofibromas, metaplastic papillary tumors, and endometrioid polyps. Cystadenofibromas, specifically, originate from invaginations of the ovarian surface epithelium, a process linked to the natural morphological changes that occur in the ovary during the reproductive years. The fibrous component of these tumors results from varying degrees of cortical stromal proliferation and collagen deposition [14]. Based on the degree of epithelial proliferation, cystadenofibromas are categorized as benign, borderline, or malignant, although malignant transformation is exceedingly rare [4].
These tumors exhibit three primary morphological patterns on imaging: multilocular solid lesions with a “black sponge” appearance, unilocular cystic lesions with parietal thickening, and purely cystic masses. Imaging features, such as T2-weighted hypointensity (referred to as the “dark-dark appearance”), absence of diffusion restriction, and progressive post-contrast enhancement (type I perfusion curve), are characteristic. However, these features can lead to misdiagnosis as malignancies due to their solid components or irregular septations [4, 6, 15]. Tumors that often mimic ovarian cystadenofibromas include fibromas, Brenner tumors, struma ovarii, highly fibrous metastatic ovarian tumors (especially from the gastrointestinal tract), and endometriomas [4, 16]. Furthermore, the other differentials for bilateral ovarian masses include Krukenberg’s tumor [17], ovarian tuberculosis [18, 19], endometriomas [20], and very rarely polycystic ovarian disease [21].
Macroscopically, serous cystadenofibromas vary significantly in size, ranging from an average of 5–8 cm to as large as 30 cm in diameter. Larger tumors pose a notable risk for ovarian torsion, particularly due to their ability to grow substantially [16]. Accurate diagnosis requires a combination of imaging and histopathological correlation. Complete surgical excision is the preferred treatment, with most cases yielding an excellent prognosis when managed appropriately [5].
In contrast, ovarian collision tumors consist of epithelial, germ cell, and sex-cord-stromal tumor combinations [12]. The most common combination involves mucinous cystadenoma coexisting with other tumor types, such as Brenner tumor, mature cystic teratoma, Sertoli-Leydig cell tumor, or serous cystadenoma [22, 23]. The pathogenesis of collision tumors remains unclear, with proposed hypotheses ranging from the simultaneous proliferation of distinct cell lines to differentiation from a common pluripotent precursor stem cell [9]. Other theories suggest mechanisms such as [24–26]:
- Chance Occurrence: Collision tumors may arise from the coincidental, simultaneous proliferation of two distinct cell lines (“chance accidental meeting”).
- Microenvironmental Influence: The presence of the first tumor could alter the local microenvironment— through mechanisms such as angiogenesis and inflammation—promoting the development of a second primary tumor or facilitating the seeding of metastatic cells.
- Carcinogenic Exposure: A carcinogenic agent interacting with different tissues may induce the formation of distinct tumors.
- Proximity of Origin: The occurrence of two different histological subtypes may be a result of the tumors arising independently from neighboring tissues.
While none of these theories fully explain all cases, they offer insight into the complex mechanisms that may contribute to the development of ovarian collision tumors [24, 25].
Seromucinous neoplasms, a newly recognized category of ovarian epithelial tumors introduced in the 2014 revised WHO classification, further expand the spectrum of ovarian tumors. These rare tumors, derived from Müllerian tissue, are classified into benign, borderline, and malignant categories. They are characterized by a unique combination of cell types, including endocervical-type mucinous, endometrioid, and squamous epithelial elements [13].
Recent case reports have documented the coexistence of serous and mucinous cystadenomas within a single ovary, underscoring the rarity of such findings [23].
Most collision tumors are typically diagnosed postoperatively through histopathological examination. Currently, there are no standardized management protocols for collision tumors, and treatment is largely guided by the tumor’s specific components. In clinical practice, both management strategies and prognosis depend on several key factors, including the histological types of the colliding components, the most aggressive component present, and the stage of the malignant tumor [9, 27]. Benign tumors are generally managed with surgical excision alone, while malignant components often require a combination of surgery, radiation therapy, and chemotherapy. Pelvic and para-aortic lymph node dissection is not recommended for patients with benign ovarian tumors. Furthermore, studies indicate that in early-stage ovarian cancer, lymph node dissection does not significantly improve progression-free or overall survival rates and may contribute to increased postoperative morbidity [28–30]. In the present case, preoperative evaluation—including clinical assessment, radiological imaging, tumor marker analysis, and intraoperative findings—strongly suggested the benign nature of the ovarian masses, thereby obviating the need for pelvic lymph node dissection. Furthermore, the frozen section of the masses was not performed, as the endometrial biopsy revealed EIN, and the patient expressed a preference against conservative management. Consequently, a total abdominal hysterectomy with bilateral salpingo-oophorectomy was undertaken. Accurate preoperative diagnosis is critical for effective treatment planning and avoiding unnecessary interventions that could pose additional risks to the patient [10].
Table 1 summarizes previously reported ovarian collision tumors with diverse histological components [7, 22, 25, 26, 31–35].
CONCLUSIONS
This case underscores the rare coexistence of serous cystadenofibroma in one ovary and collision features involving serous and mucinous cysts in the contralateral ovary, a combination scarcely reported in the literature. Ovarian collision tumors, characterized by distinct tumor types without histological intermixing, and cystadenofibromas, which are benign epithelial-stromal tumors, both represent diagnostic challenges due to their variable clinical presentations and imaging features. The importance of comprehensive preoperative evaluation, including imaging and tumor marker analysis, is evident in ensuring accurate diagnosis and optimal management. Histopathological examination remains the cornerstone of definitive diagnosis, differentiating between benign and malignant components to guide appropriate treatment. In this case, surgical intervention successfully managed the lesions, resulting in an excellent prognosis.
AUTHOR CONTRIBUTIONS
N. Kumar: conceptualization, literature search, data collection, formal analysis, writing original drafts, writing review and editing, final review, and approval of the manuscript. A. Rath: data collection, formal analysis, writing and editing, final review, and approval of the manuscript. A. Setty: data collection, formal analysis, writing and editing, final review, and approval of the manuscript. P. T. Rathod: data collection, formal analysis, writing and editing, final review, and approval of the manuscript. J. Aparna: data collection, formal analysis, writing and editing, final review, and approval of the manuscript.
ACKNOWLEDGMENTS
I thank Mrs. Amrita Kumar, Dr. Namit Singh, Adhvan Singh, Nutty Singh, and Lexi Singh for their constant support and motivation.
CONFLICTS OF INTEREST
Authors have no conflicts of interest to declare.
ETHICAL STATEMENT
Not applicable (As per the Institution’s policy for case reports, ethical clearance is not required.)
CONSENT
The case report was conducted after the patient provided informed written consent for data publication.
FUNDING
No funding was used for this paper.
- 1. Ovarian serous cystadenofibroma: A rare case report. Asian J Surg. 2024; 47:3246–47. https://doi.org/10.1016/j.asjsur.2024.03.071. PMID:38521757
- 2. Serous Papillary Cystadenofibroma of Ovary: A Rare Case Report. J Obstet Gynaecol India. 2024; 1–3. http://dx.doi.org/10.1007/s13224-024-01976-8.
- 3. Pitfalls of Frozen Section in Gynecological Pathology: A Case of Ovarian Serous Surface Papillary Adenofibroma Imitating Malignancy. Int J Surg Pathol. 2019; 27:268–70. https://doi.org/10.1177/1066896918818894. PMID:30563377
- 4. Pearls and Potential Pitfalls for Correct Diagnosis of Ovarian Cystadenofibroma in MRI: A Pictorial Essay. Korean J Radiol. 2021; 22:1809–21. https://doi.org/10.3348/kjr.2020.1312. PMID:34668348
- 5. Serous cystadenofibroma misdiagnosed as an ovarian malignancy. BMJ Case Rep. 2018; 11:e228223. https://doi.org/10.1136/bcr-2018-228223. PMID:30567152
- 6. Serous ovarian cystadenofibroma and review of the literature: Report of a case. Int J Surg Case Rep. 2023; 110:108649. https://doi.org/10.1016/j.ijscr.2023.108649. PMID:37639968
- 7. Ovarian collision tumour consisting of a fibroma and a serous cystadenoma: A case report. Case Rep Womens Health. 2024; 42:e00602. https://doi.org/10.1016/j.crwh.2024.e00602. PMID:38577170
- 8. Ovarian Collision Tumor, Massive Mucinous Cystadenoma, and Benign Mature Cystic Teratoma. Cureus. 2021; 13:e16221. https://doi.org/10.7759/cureus.16221. PMID:34367821
- 9. Collision tumours of ovary: a very rare case series. J Clin Diagn Res. 2014; 8:FD14–16. https://doi.org/10.7860/JCDR/2014/11138.5222. PMID:25584236
- 10. Ovarian collision tumor consisting of sclerosing stromal tumor and mature cystic teratoma complicated with Meigs syndrome: A case report. Oncol Lett. 2022; 24:443. https://doi.org/10.3892/ol.2022.13563. PMID:36420070
- 11. Mature Cystic Teratoma with Co-existent Mucinous Cystadenocarcinoma in the same Ovary-A Diagnostic Dilemma. J Clin Diagn Res. 2016; 10:ED11–13. https://doi.org/10.7860/JCDR/2016/22150.9118. PMID:28208869
- 12. Ovarian collision tumors: imaging findings, pathological characteristics, diagnosis, and differential diagnosis. Abdom Radiol (NY). 2018; 43:2156–68. https://doi.org/10.1007/s00261-017-1419-6. PMID:29198011
- 13. Seromucinous tumors of ovary: A case series. Indian J Pathol Oncol. 2024; 11:166–71. https://doi.org/10.18231/j.ijpo.2024.035.
- 14. Ovarian Cystadenofibroma: An Innocent Tumor Causing Early Postoperative Small Bowel Obstruction and Perforation Peritonitis. J Midlife Health. 2024; 15:43–47. https://doi.org/10.4103/jmh.jmh_5_24. PMID:38764931
- 15. Imaging in gynecological disease (16): clinical and ultrasound characteristics of serous cystadenofibromas in adnexa. Ultrasound Obstet Gynecol. 2019; 54:823–30. https://doi.org/10.1002/uog.20277. PMID:30937992
- 16. Sonographic Evaluation of Serous Cystadenofibroma With Evidence of Intermittent Torsion. J Diagn Med Sonogr. 2021; 37:283–89. https://doi.org/10.1177/8756479321989664.
- 17. Krukenberg Tumor. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2025. https://www.ncbi.nlm.nih.gov/books/NBK482284/. PMID:29489206
- 18. Abdominal Tuberculosis Mimicking Ovarian Cancer: A Case Report and Review of the Literature. Int Med Case Rep J. 2022; 15:169–85. https://doi.org/10.2147/IMCRJ.S348434. PMID:35431583
- 19. Ovarian tuberculosis mimicking ovarian malignancy in an unvaccinated patient: A case report. Int J Surg Case Rep. 2024; 122:110031. https://doi.org/10.1016/j.ijscr.2024.110031. PMID:39059239
- 20. Decidualized ovarian endometrioma mimicking malignancy in pregnancy: a case report and literature review. J Ovarian Res. 2022; 15:33. https://doi.org/10.1186/s13048-022-00966-6. PMID:35264232
- 21. Complicated giant polycystic ovary mimicking tumor: MR imaging findings. Pediatr Radiol. 2007; 37:233–36. https://doi.org/10.1007/s00247-006-0380-2. PMID:17180658
- 22. Coexisting ovarian serous cystadenoma with fibroma: A very unusual combination. J Cancer Res Ther. 2023; 19:1474–76. https://doi.org/10.4103/jcrt.jcrt_2319_21. PMID:37787335
- 23. The Coexistence of Two Different Epithelial Ovarian Tumors: A Rare Case. Curr Med Imaging. 2022; 18:421–24. https://doi.org/10.2174/1573405617666210908125237. PMID:34503421
- 24. Collision tumor: serous cystadenocarcinoma and dermoid cyst in the same ovary. Arch Gynecol Obstet. 2009; 279:767–70. https://doi.org/10.1007/s00404-008-0781-6. PMID:18818939
- 25. Collision tumor of ovary – Case report. Indian J Pathol Oncol. 2023; 10:201–3. https://doi.org/10.18231/j.ijpo.2023.043.
- 26. A rare case of collision tumour of the ovary complicated by torsion. BJR Case Rep. 2022; 7:20210114. https://doi.org/10.1259/bjrcr.20210114. PMID:35300239
- 27. Collision Tumors in Ovary: Case Series and Literature Review. J Surg Oncol. 2019; 2019:2674–3000. http://dx.doi.org/10.31487/j.JSO.2019.03.06.
- 28. Survival outcomes of lymph node dissection in early-stage epithelial ovarian cancer: identifying suitable candidates. World J Surg Oncol. 2024; 22:294. https://doi.org/10.1186/s12957-024-03571-7. PMID:39511679
- 29. The Significance of Lymph Node Dissection in Patients with Early Epithelial Ovarian Cancer. Ann Ital Chir. 2024; 95:628–35. https://doi.org/10.62713/aic.3353. PMID:39186355
- 30. Is there any therapeutic role of pelvic and para-aortic lymphadenectomy in apparent early stage epithelial ovarian cancer? Gynecol Oncol. 2021; 160:56–63. https://doi.org/10.1016/j.ygyno.2020.10.028. PMID:33168305
- 31. A Collision between Cavernous-Capillary Hemangioma with Stromal Luteinization and Serous Cystadenoma. Folia Med (Plovdiv). 2020; 62:851–55. https://doi.org/10.3897/folmed.62.e51551. PMID:33415926
- 32. A Rare Case of Collision Tumours of the Ovary: An Ovarian Serous Cystadenoma Coexisting with Fibrothecoma. Diagnostics (Basel). 2022; 12:2840. https://doi.org/10.3390/diagnostics12112840. PMID:36428899
- 33. Collision Tumor of the Ovary Involving Sertoli-Leydig Cell Tumor and High-Grade Serous Carcinoma-Report of the First Case. Int J Gynecol Pathol. 2023; 42:254–58. https://doi.org/10.1097/PGP.0000000000000896. PMID:35838626
- 34. Giant ovarian solid and cystic masses mixed with three types of tumors: A rare case report and literature review. Heliyon. 2024; 10:e35075. https://doi.org/10.1016/j.heliyon.2024.e35075. PMID:39161819
- 35. Collision Tumor of the Ovary: Adult Granulosa Cell Tumor and Mesonephric-like Adenocarcinoma. Diagnostics (Basel). 2024; 14:1412. https://doi.org/10.3390/diagnostics14131412. PMID:39001303
