Oncoscience
RAS mutations vary between lesions in synchronous primary colorectal cancer: testing only one lesion is not sufficient to guide anti-EGFR treatment decisions
Mariana Petaccia de Macedo1,2, Fernanda Machado de Melo1, Júlia da Silva Ribeiro1, Celso Abdon Lopes de Mello3, Maria Dirlei Ferreira de Souza Begnami1,2, Fernando Augusto Soares1,2, Dirce Maria Carraro1,4 and Isabela Werneck da Cunha1,2
1 Department of Molecular Diagnosis, Anatomic Pathology Department, AC Camargo Cancer Center, São Paulo, Brazil
2 Laboratory of Investigative Pathology, CIPE / AC Camargo Cancer Center, São Paulo, Brazil
3 Department of Clinical Oncology, AC Camargo Cancer Center, São Paulo, Brazil
4 Laboratory of Genomics and Molecular Biology, CIPE / AC Camargo Cancer Center, São Paulo, Brazil
Correspondence to: Mariana Petaccia de Macedo, email: [email protected]
Keywords: synchronic primary colorectal cancer; colorectal cancer, KRAS, NRAS, anti-EGFR treatment
Received: October 16, 2014
Accepted: February 06, 2015
Published: February 09, 2015
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
ABSTRACT
Introduction: Mutations in KRAS and NRAS genes are negative predictors of anti-EGFR therapies response in metastatic colorectal cancer. There are few reports on RAS testing in synchronous primary colorectal cancer (SP-CRC) and a lack of recommendations on which tissue should be tested for the mutation in this disease. This study analyzed the RAS status of both lesions in SP-CRC patients and in their metastasis. Materials and methods: DNA was obtained from formalin-fixed-paraffin-embedded tissue, and mutations were analyzed by pyrosequencing. Results: RAS status was heterogeneous in 6 (75%) of 8 SP-CRC patients between primary lesions. Five showed heterogeneity regarding RAS mutational status, and from these, four presented with metastasis: 3 cases (75%) had WT metastatic tissue, and 1 case (25%) had mutated metastatic tissue. One patient showed divergence regarding RAS mutation type. Discussion: RAS mutations vary significantly between SP-CRC lesions, and the status of the metastasis is unpredictable. Testing for RAS mutations in only 1 of the primary lesions can misguide clinical decisions and hind the predictive potential of anti-EGFR treatment. A more appropriate approach in metastatic SP-CRC is to test the metastatic tissue or both primary lesions for providing more accurate mutation scenery and support more assertive clinical decisions.
INTRODUCTION
The definition of synchronous primary colorectal carcinoma (SP-CRC) is the existence of more than one primary colorectal carcinoma (CRC) in a single patient1. This condition differs from metastatic synchronous CRC, in which metastasis is diagnosed at the time of the primary tumor [2]. SP-CRC is estimated to account for 3.5% of all CRCs [1]. SP-CRC is more common in men and is associated to predisposing conditions, such as inflammatory bowel disease, hereditary nonpolyposis CRC (Lynch Syndrome), and familial adenomatous polyposis [1].
The prognosis of SP-CRC is unknown [3, 4, 5]. Compared with solitary CRC, SP-CRC is more often associated with right-sided tumors, mucinous histology, and precursor sessile serrated adenoma (SSA). Molecularly, SP-CRC is linked to high microsatellite instability (MSI-H) and concurrent BRAF gene mutations [4], although these relationships are controversial [3].
Mutations in KRAS are well-established negative predictors of the response to anti-EFGR therapies in the treatment of metastatic CRC [6]. KRAS mutations are observed in 35% to 40% of CRCs and arise more often in codons 12 (80%) and 13 (15%) of exon 2 [7, 8, 9] and to a lesser extent in codons 61, 117, and 146 [7, 8, 9]. Unsual KRAS mutations affecting more than 1 codon and insertions have also been reported [10, 11]. Recent studies have shown that CRC patients with tumors that harbor NRAS gene mutations also have poorer response rates to EGFR inhibitors compared with those with wild-type NRAS [12]. NRAS mutations, present in approximately 5% of CRC tumors, are less frequent than KRAS mutations [13] and also developed most often in codons 61, 12, and 13. Concomitant mutations in KRAS and NRAS are a rare finding [12].
Thus, testing for KRAS and NRAS mutations is necessary before anti-EGFR therapies are initiated in CRC patients [12, 14, 15]. Concerns have been raised since the KRAS mutation testing recommendations were issued regarding the ideal tissue that should be examined. The concordance of KRAS status between primary and metastatic CRC tissue in the same patient varies significantly, with heterogeneity ranging from 0% to 31% but tending to be low [16]. Studies that compared CRC biopsies before and after neoadjuvant therapy did not report any differences regarding KRAS status [17, 18, 19], nor did studies that compared biopsy and resection specimens in CRC [20, 21, 22].
As a result, an issue has arisen regarding patients with more than one primary lesion: should RAS mutations be tested in both lesions? In the daily routine of a molecular pathology laboratory, facing that situation is not unusual, especially for those that perform high-volume RAS mutation testing.
The aim of this study was to analyze KRAS and NRAS mutational status in both lesions of SP-CRC patients as well as the metastatic tissue and determine the necessity of testing for both lesions in order to provide more precise information for supporting clinical decision.
RESULTS
Clinical and pathological data
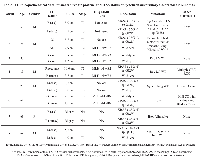
We retrieved 8 cases with SP-CRC from our molecular pathology laboratory records. The 8-patient series comprised 5 males (62.5%) and 3 females (37.5%), and the mean age was 71.5 years. All patients presented with 2 synchronous invasive CRC lesions at the time of the surgical resection (7 patients) or biopsy (1 patient). Five patients show lymph node (LN) metastasis, 4 of whom had additional systemic metastasis (patient 1- liver, pleural and abdominal; patient 2- lung and brain; patient 5- lung; and patient 8- liver). See Table 1 for their clinical and pathological data.
Molecular pathology: KRAS mutational analysis of synchronous carcinomas
Of the 16 primary tumor samples in the 8 patients, 7 had wild-type RAS and 9 had mutated RAS samples. KRAS mutation was the most frequent (8 of 9 mutations, 88%). KRAS codon 12 was the most frequently mutated codon (7 of 9 mutated samples, 77%). Three mutations were noted: 3 cases of c.35G>A in KRAS codon 12 (p.G12D), 3 cases of c.35G>T (p.G12V) in KRAS codon 12, and 1 case of c.34G>A (p.G12S). A KRAS mutation in codon 13 (c.38G>A [p.G13D]) was observed in 1 patient, and a NRAS mutation in codon 61 (c.182A>T [p.Q61L]) detected in 1 patient. There were no mutations in KRAS codons 61, 117, or 146 or in NRAS codons 12, 13, 117, or 146.
RAS mutations were conflicting in 6 (75%) of the 8 SP-CRC patients analyzed in this study. Conflicts were regarding RAS status and RAS mutation type. Five patients (83% and 62.5% of the heterogeneous or whole study group, respectively) (patients 2, 4, 5, 6, and 8) had 1 lesion with wild-type (WT) RAS and 1 lesion with mutated RAS; both lesions in the remaining patient (patient 1) harbored a mutation in KRAS codon 12 c.35G>A (p.G12D) and c.34G>A (p.G12S), respectively. Of the 2 cases that showed no heterogeneity with regard to RAS mutation between both primary CRC lesions, patient 3 had WT RAS in both lesions, and patient 7 had the c.35G>T KRAS mutation in both lesions.
Seven of the 8 patients had LN or systemic metastasis. We noted several profiles of LN and systemic metastatic tissue in patients with heterogeneous RAS mutation status in the primary lesions. Of the 4 metastatic cases with both WT and mutated RAS status, 3 (75%) (patients 4, 5, and 8) and 1 (25%) (patient 2) resulted in the metastatic tissue RAS WT and mutated, respectively. In the patient with disparate RAS mutations in the primary lesions (patient 1), both the LN and liver metastases had the same KRAS c.34G>A (p.G12S) mutation. See Table 1 for RAS mutational data.
DISCUSSION
In this study, we evaluated the RAS mutational status of both lesions in 8 patients with SP-CRC and found that RAS mutations are commonly heterogeneous between SP-CRC lesions.
The rate of heterogeneity between lesions was 75% with regard to RAS mutational status and type. Although some studies have indicated that specific KRAS mutations respond to EGFR inhibitors[24], specially p.G13D, RAS-mutated tumors generally fail to respond to anti-EGFR treatment, regardless of the nucleotide substitution. If we consider only cases with heterogeneity in RAS mutational status (WT and mutated), 62.5% of SP-CRC cases showed clinically relevant heterogeneity of RAS mutational status between primary tumors.
Previous studies have reported molecular heterogeneity of both lesions in SP-CRC. Eguchi and colleagues [25] analyzed p53 mutations in both lesions of 16 SP-CRC and found that 7 patients harbored a p53 mutation in only 1 lesion. In 9 patients, both lesions were mutated, but the mutations always differed between lesions from the same patient. Thus, regarding the p53 mutational status in SP-CRC, the authors found no concordance in p53 mutation status between lesions, suggesting that the synchronous tumors had a multicentric, not monoclonal, origin. Another group [5] showed that the pattern of CpG island methylation was concordant in synchronous cancer pairs in the same location in the colon (proximal-proximal) and colorectum (distal-distal) but not in tumor pairs in differing locations (eg, 1 proximal cancer and 1 distal cancer).
Previous studies have reported a significant percentage of discordance in KRAS mutational status between both lesions in SP-CRC. Balschun et al.studied 20 patients with SP-CRC for mutations in KRAS, NRAS, PIK3CA, and BRAF. KRAS mutations were discordant between synchronous lesions in 6 patients: 3 patients had mutated versus wild-type KRAS, and 3 patients had disparate mutation types in the synchronous lesions. NRAS status was heterogeneous in 1 patient with 4 primary lesions, only 2 of which harbored an NRAS mutation. They also tested the metastatic tissue and reported the ability to predict the origin of a metastasis by comparing the type of mutation between primary lesions [26].
Ogino et al. analyzed 6 SP-CRC patients regarding MSI, KRAS and BRAF status, and successful sequenced KRAS gene of 5 pairs of lesions. They found 3 out of 5 pairs to be discordant with regard to KRAS mutation (60%). Two cases showed discordance regarding KRAS status (WT versus mutated) and one case showed different types of mutation between paired lesions. Metastatic tissue was not tested. Further, they noted discordance for BRAF mutation status between paired lesions (p.V600E and WT status) and MSI status in 1 patient [27].
Konishi et al. evaluated 27 synchronous CRC cases and found 10 patients with discordance regarding KRAS mutational status (wild-type versus mutated). The authors did not report any data regarding the type of mutation, thus, the discordance rate might be underestimated if we consider the possibility that KRAS mutation type differed between lesions [28].
Bae et al. studied 98 lesions from 46 patients with SP-CRC and showed that KRAS mutation rates did not differ statistically between synchronous and solitary CRC, and also stated that KRAS and BRAF mutation status were not concordant in either of the synchronous pairs [29].
Koness et al. compared KRAS mutations in 15 SP-CRC patients and found 7 cases with differences in KRAS mutational status between paired tumors but did not show data regarding the type of mutation. Of the 8 cases with similar KRAS status, 1 had a mutation in both lesions [30].
One group [31] compared RAS, BRAF, PIK3CA, and TP53 status between 84 pairs of primary CRC and liver metastases and found a concordant rate of 97.6%, 98.8%, and 92.8% for RAS/BRAF, PIK3CA, and TP53, respectively. Regarding the 2 discordant KRAS mutation cases, 1 case was actually SP-CRC operated in different times with liver metastasis, and the second case was a patient with mucinous CRC and nonmucinous liver metastasis, with no additional clinical information, which were demonstrated to have developed from different primary lesions.
Collectively, our data and those of previous studies have shown that KRAS and NRAS mutations vary widely between SP-CRC lesions and that the status of the corresponding metastasis is unpredictable. Testing for KRAS mutation in only 1 of the primary lesions in SP-CRC might yield an incomplete profile on KRAS and NRAS mutation status and can misguide the clinical decision with regard to anti-EGFR treatment.
The best approach to guide anti-EGFR treatment decision in SP-CRC with metastatic disease would be to test the metastatic tissue for KRAS and NRAS mutations, because it is not possible to be certain which primary SP-CRC lesion led to the metastasis without any additional study. In clinical scenarios of impossibility in obtaining the metastatic tissue, or if patients present with multiple metastases and examining all metastatic sites is not suitable, or for SP-CRC cases in a routine molecular pathology laboratory with no additional clinical information, both primary lesions should be tested.
MATERIAL AND METHODS
Study population and histopathological features
Study participants were drawn from an institutional database between 2009 and 2014 and comprised patients of both genders and of all ages with a diagnosis of SP-CRC who were operated or biopsied on at AC Camargo Cancer Center, São Paulo, Brazil, and those who were being followed at our institution after tumor resection by an outside service and had their slides reviwed by our service. The SP-CRC cases in this study had invasive CRC lesions in the same surgical specimen. Pathological data were retrieved from the surgical pathology reports. The tumors were staged per the TNM, 7th edition [23].
Tissue samples and DNA isolation
Five 5-µm sections from formalin-fixed paraffin-embedded tissue (FFPET) blocks of 1 tumor area of both invasive adenocarcinomas and the metastatic tissue were obtained from the paraffin block. Posterior deparaffinization was performed, and tumor samples were obtained by scraping the neoplastic tissue from the glass slide (macrodissection). The representative tumor area of each case was selected by experienced pathologist, and the minimum of 30% of tumor cell in each selected area was necessary to consider a case suitable for DNA extraction. Genomic DNA was isolated using the QIAamp Kit (Qiagen).
KRAS and NRAS mutation analysis
KRAS and NRAS mutations were analyzed in 1 area of both lesions in SP-CRC patients, as well as in the LNs and systemic metastatic lesions. First, KRAS codons 12 and 13 were tested, and if they were wild-type, mutations in KRAS codon 61 and NRAS codons 12, 13, and 61 were examined. If the sample remained wild-type for the tested codons, then, KRAS and NRAS codons 117 and 146 were analyzed.
Mutations were evaluated by pyrosequencing per the manufacturer’s instructions [KRAS PyroMarkTM Q24 kit, NRAS Pyro Kit, RAS extension KIT (Qiagen)]. Ten microliters of biotinylated PCR product was conjugated to streptavidin-sepharose beads (GE Healthcare) per a standard protocol for single-strand preparation. Pyrosequencing was performed using the PyroMarkTM Gold Q24 reagent kit (Qiagen). A cutoff value of 5% was used to define a case as positive.
Ethics committee review
This study is part of a scientific project approved by the local ethics committee (AC Camargo Cancer Center) (number1543/11, dated April 12, 2011).
Guarantor of the article
Mariana Petaccia de Macedo
Specific author contributions
Mariana Petaccia de Macedo- performed the mutation assays, analyzed the data, and wrote the manuscript. The author approves the final submitted draft.
Fernanda Machado de Melo performed the mutations assays and analyzed the data. The author approves the final submitted draft.
Júlia da Silva Ribeiro performed the mutation assays and analyzed the data. The author approves the final submitted draft.
Celso Abdon Lopes de Mello analyzed the data and reviewed the manuscript. The author approves the final submitted draft.
Maria Dirlei Begnami analyzed the data and wrote the manuscript. The author approves the final submitted draft.Fernando Augusto Soares analyzed the data and reviewed the manuscript. The author approves the final submitted draft.
Dirce Maria Carraro analyzed the data and reviewed the manuscript. The author approves the final submitted draft.Isabela Werneck da Cunha analyzed the data and wrote the manuscript. The author approves the final submitted draft.
Financial support
We acknowledge Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, grant 2011/ 08510-2) for financial support and Fundação Antônio Prudente – AC Camargo Cancer Center, São Paulo, SP, Brazil, for institutional support.
Potential competing interests
We declare no conflict of interests.
- 1. Synchronous colorectal cancer: Clinical, pathological and molecular implications. WJG. 2014;20:6815. https://doi.org/10.3748/wjg.v20.i22.6815. [PubMed].
- 2. Influence of KRAS mutation status in metachronous and synchronous metastatic colorectal adenocarcinoma. Cancer. 2012;118:6243–52. https://doi.org/10.1002/cncr.27666. [PubMed].
- 3. Molecular heterogeneity and prognostic implications of synchronous advanced colorectal neoplasia. Br J Cancer. 2014;110:1228–35. https://doi.org/10.1038/bjc.2013.827. [PubMed].
- 4. Clinicopathologic features of synchronous colorectal carcinoma: A distinct subset arising from multiple sessile serrated adenomas and associated with high levels of microsatellite instability and favorable prognosis. Am J Surg Pathol. 2013;37:1660-70. https://doi.org/10.1097/PAS.0b013e31829623b8. [PubMed].
- 5. A Prospective Cohort Study Shows Unique Epigenetic, Genetic, and Prognostic Features of Synchronous Colorectal Cancers. Gastroenterology. 2009;137:1609–1620. https://doi.org/10.1053/j.gastro.2009.08.002. [PubMed].
- 6. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408-17. https://doi.org/10.1056/NEJMoa0805019. [PubMed].
- 7. K-ras oncogene mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis. 2003; 24:703-10. https://doi.org/10.1093/carcin/bgg009. [PubMed].
- 8. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9:1193-7. [PubMed].
- 9. P. and J. L. Hunt. KRAS mutation testing in colorectal cancer. Adv Anat Pathol. 2009; 16:196-203. https://doi.org/10.1088/1361-648X/ab243d. [PubMed].
- 10. Multiple mutations in the Kras gene in colorectal cancer: review of the literature with two case reports. Int J Colorectal Dis. 2011;26:1241-8. https://doi.org/10.1007/s00384-011-1238-0. [PubMed].
- 11. de Macedo MP, de Lima LG, Begnami MD, de Melo FM, Andrade LD, Lisboa BC, Soares LM, Soares FA, Carraro DM, da Cunha IW. KRAS insertions in colorectal cancer: what do we know about unusual KRAS mutations? Exp Mol Pathol. 2014;96:257-60. https://doi.org/10.1016/j.yexmp.2014.02.014. [PubMed].
- 12. Panitumumab–FOLFOX4 Treatment and RASMutations in Colorectal Cancer. N Engl J Med. 2013;369:1023–34. https://doi.org/10.1056/NEJMoa1305275. [PubMed].
- 13. NRAS Mutations Are Rare in Colorectal Cancer. Diagnostic Molecular Pathology. 2010;19:157–63. https://doi.org/10.1097/PDM.0b013e3181c93fd1. [PubMed].
- 14. Allegra CJ, Jessup JM, Somerfield MR, Hamilton SR, Hammond EH, Hayes DF, McAllister PK, Morton RF, Schilsky RL. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091-6. https://doi.org/10.1200/JCO.2009.21.9170. [PubMed].
- 15. KRAS mutation testing for predicting response to anti-EGFR therapy for colorectal carcinoma: proposal for an European quality assurance program. Virchows Archiv. 2008;453:417–431. https://doi.org/10.1007/s00428-008-0665-y. [PubMed].
- 16. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. British Journal of Cancer. 2011;104:1020–1026. https://doi.org/10.1038/bjc.2011.26. [PubMed].
- 17. Ondrejka SL, Schaeffer DF, Jakubowski MA, Owen DA, Bronner MP. Does neoadjuvant therapy alter KRAS and/or MSI results in rectal adenocarcinoma testing? Am J Surg Pathol. 2011 Sep;35(9):1327-30. https://doi.org/10.1097/PAS.0b013e3182253800. [PubMed].
- 18. KRAS genotyping in rectal adenocarcinoma specimens with low tumor cellularity after neoadjuvant treatment. Mod Pathol. 2012;25:731-9. https://doi.org/10.1038/modpathol.2011.210. [PubMed].
- 19. KRAS mutations in primary tumours and post-FOLFOX metastatic lesions in cases of colorectal cancer. Br J Cancer. 2012 Jul 10;107:340-4. https://doi.org/10.1038/bjc.2012.218. [PubMed].
- 20. The utility of diagnostic biopsy specimens for predictive molecular testing in colorectal cancer. Histopathology. 2012;61:1117-24. https://doi.org/10.1111/j.1365-2559.2012.04321.x. [PubMed].
- 21. Concordance in KRAS and BRAF mutations in endoscopic biopsy samples and resection specimens of colorectal adenocarcinoma. Eur J Cancer. 2012;48:1108-15. https://doi.org/10.1016/j.ejca.2012.02.054. [PubMed].
- 22. KRAS mutational status of endoscopic biopsies matches resection specimens. J Clin Pathol. 2012;65:604-7. https://doi.org/10.1136/jclinpath-2012-200746. [PubMed].
- 23. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL and Trotti A. AJCC Cancer Staging Manual. Springer: New York, 2009.
- 24. Association of KRAS G13D tumor mutations with outcome in patients with metastatic colorectal cancer treated with first-line chemotherapy with or without cetuximab. J Clin Oncol. 2012 ;30:3570-7. https://doi.org/10.1200/JCO.2012.42.2592. [PubMed].
- 25. Discordance of p53 mutations of synchronous colorectal carcinomas. Mod Pathol. 2000 Feb;13:131-9. https://doi.org/10.1038/modpathol.3880024. [PubMed].
- 26. KRAS, NRAS, PIK3CA exon 20, and BRAF genotypes in synchronous and metachronous primary colorectal cancers diagnostic and therapeutic implications. J Mol Diagn. 2011;13:436-45. https://doi.org/10.1016/j.jmoldx.2011.03.002. [PubMed].
- 27. Epigenetic profiling of synchronous colorectal neoplasias by quantitative DNA methylation analysis. Mod Pathol. 2006;19:1083-90. https://doi.org/10.1038/modpathol.3800618. [PubMed].
- 28. Concordant DNA methylation in synchronous colorectal carcinomas. Cancer Prev Res (Phila). 2009;2:814-22. https://doi.org/10.1158/1940-6207.CAPR-09-0054. [PubMed].
- 29. Clinicopathologic and molecular characteristics of synchronous colorectal cancers: heterogeneity of clinical outcome depending on microsatellite instability status of individual tumors. Dis Colon Rectum. 2012 Feb;55:181-90. https://doi.org/10.1097/DCR.0b013e31823c46ce. [PubMed].
- 30. Koness RJ, King TC, Schechter S, McLean SF, Lodowsky C, Wanebo HJ. Synchronous colon carcinomas: molecular-genetic evidence for multicentricity. Ann Surg Oncol1996;3:136-43. https://doi.org/10.1007/BF02305792. [PubMed].
- 31. Comparative genomic analysis of primary versus metastatic colorectal carcinomas. J Clin Oncol. 2012;30:2956-62. https://doi.org/10.1200/JCO.2011.38.2994. [PubMed].
Last Modified: 2016-06-06 23:40:21 EDT

